Chars play an important role as black pigments. Well known are pieces of charcoal, charred wood, which can often be found in the ashes of wood-fueled fires. Deliberate production of charcoal was a precondition for the ability to smelt iron ore.[1] Charring is a specific process of thermal decomposition, irreversibly changing the chemical and physical properties of organic materials.
The charring process differs essentially from soot production. During charring, oxygen is deliberately excluded with the aim of manipulating the combustion process (anoxic conditions). This is realized by heating the raw material in a nearly air-tight environment, for instance by putting the raw material into a fire-proof crucible which is placed in a fire (Fig. 19a). Another process is the ancient technique of large-scale production of charcoal where a closely-stacked wood pile is covered with a layer of muddy clay and ignited internally (Figure 19b). It needs to be taken into account that during charring many gasses are released which must be able to escape, so the process cannot be completely air-tight. The charring process passes four phases:

> Drying: At 100°C the not-chemically bound water evaporates.
> Thermal decomposition: Once a temperature of 280°C – 300°C is reached, organic components undergo a rapid thermal decomposition, chemical bonds break and non-carbon elements like hydrogen are released from the carbon chains and form gasses which escape, causing a considerable loss of mass. These chemical reactions continue with increased temperatures, but at a lower rate. The thermal decay is specific for different materials, like for instance bone[2] or wood.[3] Flame formation and further combustion are impeded by the absence of oxygen.
> Carbonization: The remaining material, which cannot further incinerate, starts to change its chemical structure, a process called carbonization. During that stage, the carbon framework starts to reorganize.
> Cooling: Finally, during the cooling phase, the carbon re-crystallizes. It forms a graphite-like structure that causes an increased absorption of electromagnetic radiation, and hence the material appears black.[4]
After charring, the outer structure of the material remains unchanged, but is shrunken due to the loss of organic matter and the color is black. All inorganic components remain, and play an important role in scientific identification of black char-based pigments.[5] Depending on the initial strength of the material, charred structures can be quite solid and need pounding in a mortar and thorough grinding before they are suitable as pigments.
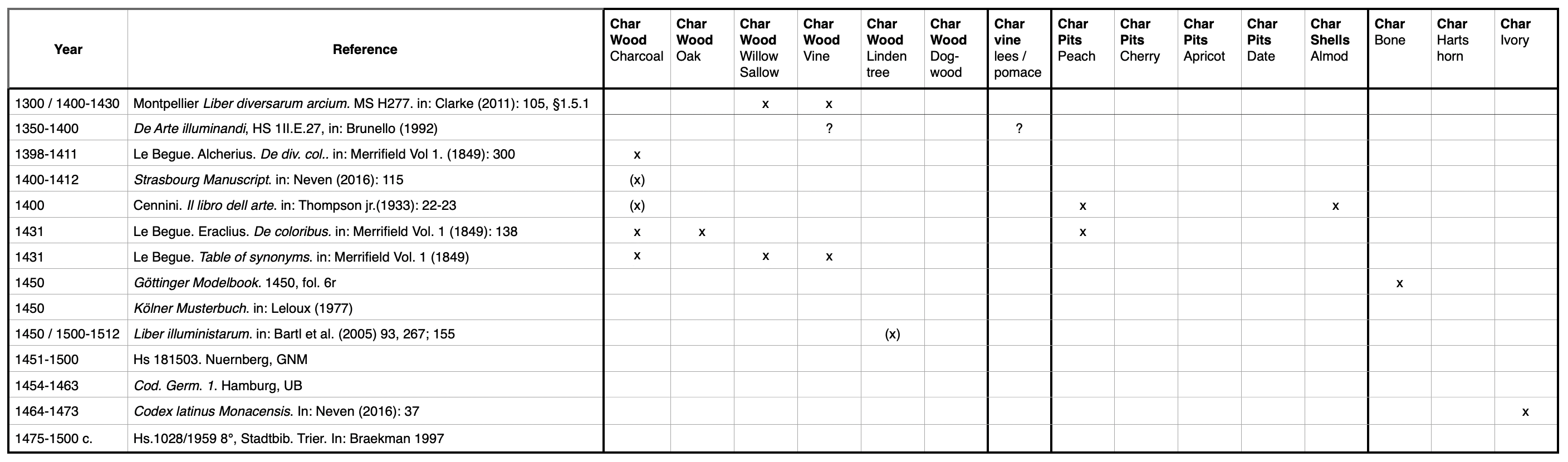
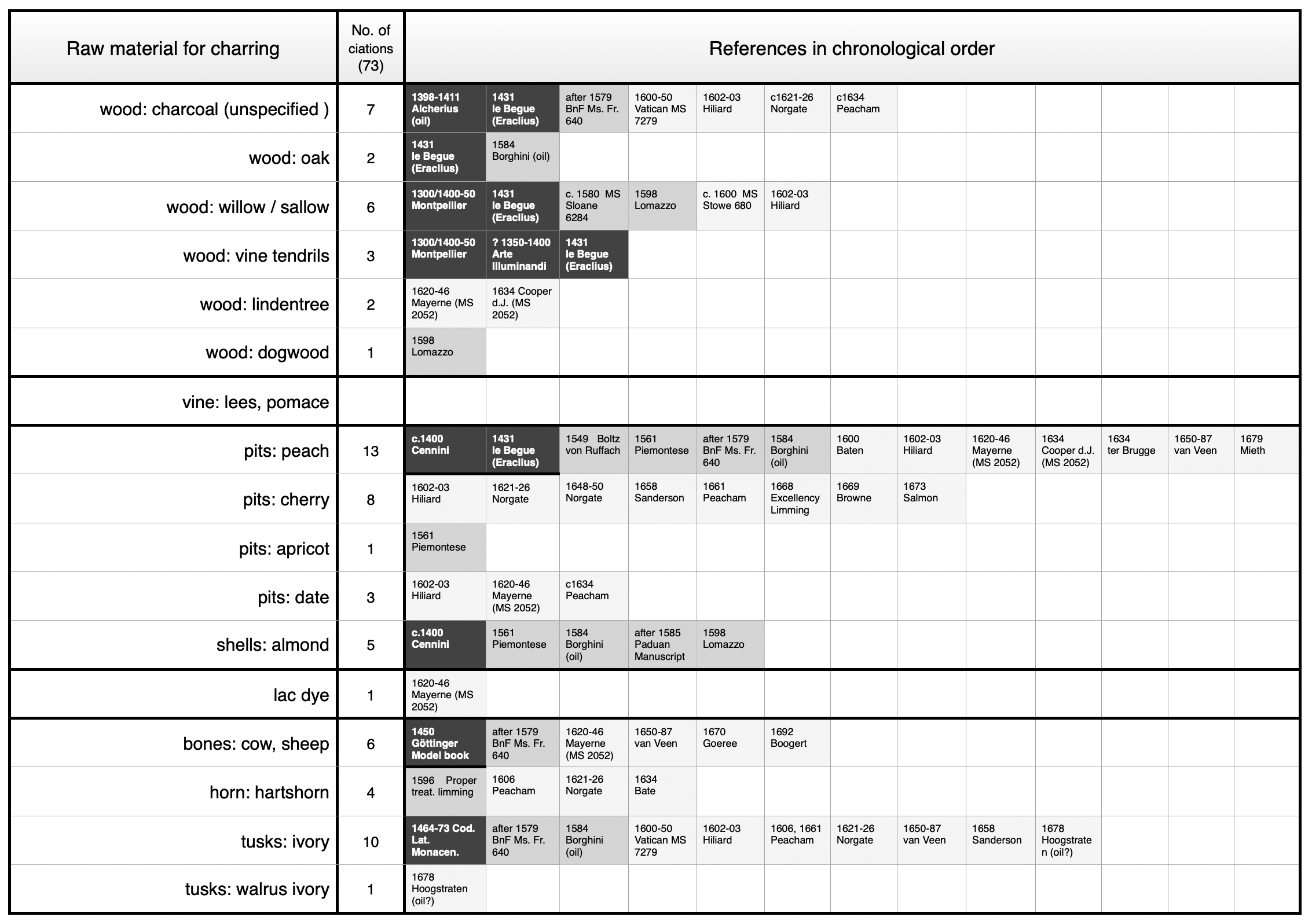
According to historic recipes, four major groups of charred materials can be distinguished: (1) wood, (2) shells and pits of nuts and fruits, (3) bones, horn, ivory, and (4) incompletely burnt materials.
[1] Joosten. 2004. Technology of Early Historical Iron Production in the Netherlands. Dissertation: p. 133.
[2] Reidsma et al. 2016. Charred Bone: p. 282 – 292.
[3] Braadbaart and Poole. 2008. Morphological, Chemical and Physical Changes during Charcoalification of Wood: p. 2434 – 2445.
[4] Chylek et al. 2015. Soot: p. 57.
[5] Depending on the plant and the soil as a major source of nutrients, characteristic elements remain after charring, for instance potassium, aluminum and magnesium as well as trace elements. For animal-based chars, calcium and phosphor are marker elements.
[1] Joosten. 2004. Technology of Early Historical Iron Production in the Netherlands. Dissertation: p. 133.
[2] Reidsma et al. 2016. Charred Bone: p. 282 – 292.
[2] Reidsma et al. 2016. Charred Bone: p. 282 – 292.
[4] Chylek et al. 2015. Soot: p. 57.
[4] Chylek et al. 2015. Soot: p. 57.